Breakthrough Research in Crohn's Disease Treatment – New Opportunity Brings Hope to Patients!
Bệnh Crohn, một thể của bệnh viêm ruột mạn tính ngày càng trở nên phổ biến, gây ra nhiều biến chứng nghiêm trọng như rò, thủng ruột, apxe ổ bụng…. ảnh hưởng đến sức khỏe và chất lượng cuộc sống của người bệnh. Mặc dù đã có nhiều tiến bộ trong phương pháp điều trị như thuốc sinh học và thuốc phân tử nhỏ, nhưng vẫn còn nhiều người bệnh không đáp ứng với các liệu pháp này.
AZD7798 – A new injectable monoclonal antibody designed to reduce the effects of inflammatory CCR9+ T cells, helping to reduce symptoms and improve health for people with Crohn's disease.
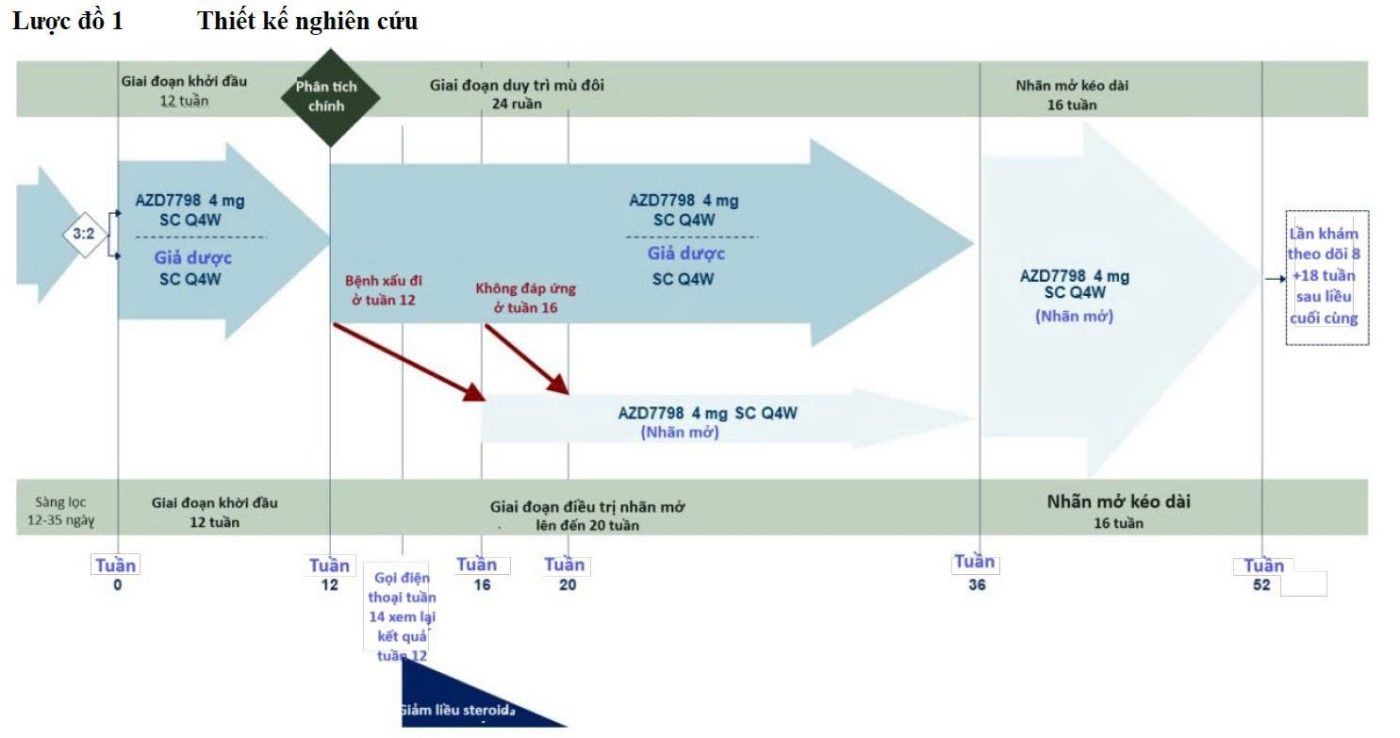
Present, AZD7798 is in phase I clinical trials and entering phase IIa (the AMALTHEA study), to evaluate efficacy and safety in patients with moderate to severe Crohn's disease.
Patients who are eligible to participate in the study will receive close medical care and monitoring and receive financial support during screening and treatment.
If you or a loved one is suffering from Crohn's disease and meets the criteria, contact us now to be selected for treatment under the program.
Criteria for selecting patients to participate in the study:
- Diagnosis of moderate to severe Crohn's disease:
- CDAI score of 220–450, or 200–450 for patients with ileal/ileocecal symptoms, and
- SES-CD ≥ 6, or SES-CD ≥ 4 for ileal/ileocecal disease.
- Have a history:
- Intolerance or inadequate response to oral corticosteroids, azathioprine, 6-mercaptopurine, methotrexate, biologics, or other approved therapies (eg, JAK inhibitors), or
- Corticosteroid dependence (defined as inability to reduce dose below 6 mg/day budesonide or 10 mg/day prednisolone without disease relapse).
Exclusion criteria:
- Colonic stenosis that the colonoscope cannot pass through.
- Surgery planned before the end of the study.
- Had more than 2 bowel surgeries or had at least one colectomy.
- Abdominal surgery within 3 months before screening, including bowel resection, bypass surgery, or colostomy.
- Nonhealing fistula or undrained abscess, including intra-abdominal abscess. Cutaneous and perianal/perianal abscesses were not exclusion criteria if appropriately treated and drained at least 4 weeks prior to study entry, and no surgery was planned.
- Oral Crohn's disease with symptoms within the past year is excluded, except for aphthous ulcers that do not require specialist intervention.
Currently, the Gastroenterology and Hepatobiliary Center of Bach Mai Hospital has screened the first patient for the AMALTHEA study and is also the first patient of the AMALTHEA study in Vietnam.
We are looking for qualified patients to participate in the research and development of this groundbreaking treatment. Contact us today!
Contact details:
Dr. Khuc Thu Trang 088 8924036
Dr. Tran Thi Kieu Oanh 035 7460810